Abstract
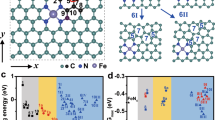
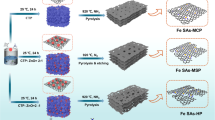
Regulating the site density of single-atom catalysts (SACs) promotes the potential to remarkably improve the performance of electrocatalysis, such as the oxygen reduction reaction (ORR). However, the catalytic behaviour governed by individual and interacting sites is particularly elusive and has yet to be understood. Here we demonstrate the origin of the enhancement of the ORR activity of isolated Fe–N4 SACs over inter-site distances down to the subnanometre level. Strong interactions between adjacent Fe–N4 moieties alter the electronic structure when the inter-site distance is less than about 1.2 nm, resulting in increased intrinsic ORR activity. The marked improvement in site performance continues until neighbouring Fe atoms approach as close as about 0.7 nm, below which the intrinsic activity is slightly diminished. The present study highlights the significance of identifying the fundamental mechanism of the inter-site distance effect in Fe–N4 catalysts for the ORR, which may promote the full potential of densely populated SACs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings in this paper are available within the article and its Supplementary Information or from the corresponding authors on reasonable request.
References
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Peng, Y., Lu, B. & Chen, S. Carbon-supported single atom catalysts for electrochemical energy conversion and storage. Adv. Mater. 30, 1801995 (2018).
Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018).
Jin, Z. & Bard, A. J. Atom-by-atom electrodeposition of single isolated cobalt oxide molecules and clusters for studying the oxygen evolution reaction. Proc. Natl Acad. Sci. USA 117, 12651–12656 (2020).
Martinez, U. et al. Progress in the development of Fe-based PGM-free electrocatalysts for the oxygen reduction reaction. Adv. Mater. 31, 1806545 (2019).
Wan, C., Duan, X. & Huang, Y. Molecular design of single-atom catalysts for oxygen reduction reaction. Adv. Energy Mater. 10, 1903815 (2020).
Zhao, L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 10, 1278 (2019).
Wang, J., Li, Z., Wu, Y. & Li, Y. Fabrication of single-atom catalysts with precise structure and high metal loading. Adv. Mater. 30, 1801649 (2018).
Ji, S. et al. Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Cichocka, M. O. et al. A porphyrinic zirconium metal–organic framework for oxygen reduction reaction: tailoring the spacing between active-sites through chain-based inorganic building units. J. Am. Chem. Soc. 142, 15386–15395 (2020).
Sours, T., Patel, A., Nørskov, J., Siahrostami, S. & Kulkarni, A. Circumventing scaling relations in oxygen electrochemistry using metal–organic frameworks. J. Phys. Chem. Lett. 11, 10029–10036 (2020).
Guo, Y. et al. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 120, 7642–7707 (2020).
Fang, Z., Li, P. & Yu, G. Gel electrocatalysts: an emerging material platform for electrochemical energy conversion. Adv. Mater. 32, 2003191 (2020).
Zhao, F., Bae, J., Zhou, X., Guo, Y. & Yu, G. Nanostructured functional hydrogels as an emerging platform for advanced energy technologies. Adv. Mater. 30, 1801796 (2018).
Zhang, N. et al. High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 13, 111–118 (2020).
Li, X., Rong, H., Zhang, J., Wang, D. & Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 13, 1842–1855 (2020).
Zhang, J. et al. Controlling N-doping type in carbon to boost single-atom site Cu catalyzed transfer hydrogenation of quinoline. Nano Res. 13, 3082–3087 (2020).
Kaiser, S. K., Chen, Z., Fuast Akl, D., Mitchell, S. & Pérez-Ramίrez, J. Single-atom catalysts across the periodic table. Chem. Rev. 120, 11703–11809 (2020).
Brown, A. P., Hillier, S. & Brydson, R. M. D. Quantification of Fe-oxidation state in mixed valence minerals: a geochemical application of EELS revisited. J. Phys. Conf. Ser. 902, 012016 (2017).
Li, P. et al. Supramolecular confinement of single Cu atoms in hydrogel frameworks for oxygen reduction electrocatalysis with high atom utilization. Mater. Today 35, 78–86 (2020).
Li, P., Jin, Z., Fang, Z. & Yu, G. A surface-strained and geometry-tailored nanoreactor that promotes ammonia electrosynthesis. Angew. Chem. Int. Ed. 59, 22610–22616 (2020).
Jin, Z. & Bard, A. J. Surface interrogation of electrodeposited MnOx and CaMnO3 perovskites by scanning electrochemical microscopy: probing active sites and kinetics for the oxygen evolution reaction. Angew. Chem. Int. Ed. 60, 794–799 (2021).
Li, P. et al. Probing enhanced site activity of Co–Fe bimetallic subnanoclusters derived from dual cross-linked hydrogels for oxygen electrocatalysis. ACS Energy Lett. 4, 1793–1802 (2019).
Jia, Q. et al. Experimental observation of redox-induced Fe–N switching behavior as a determinant role for oxygen reduction activity. ACS Nano 9, 12496–12505 (2015).
Inaba, M. et al. Benchmarking high surface area electrocatalysts in a gas diffusion electrode: measurement of oxygen reduction activities under realistic conditions. Energy Environ. Sci. 11, 988–994 (2018).
Gao, J. & Liu, B. Progress of electrochemical hydrogen peroxide synthesis over single atom catalysts. ACS Mater. Lett. 2, 1008–1024 (2020).
Ramaswamy, N., Tylus, U., Jia, Q. & Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: linking surface science to coordination chemistry. J. Am. Chem. Soc. 135, 15443–15449 (2013).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Zhang, N. et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 3, 509–521 (2020).
Yuan, K. et al. Synergetic contribution of boron and Fe–Nx species in porous carbons toward efficient electrocatalysts for oxygen reduction reaction. ACS Energy Lett. 3, 252–260 (2018).
Yamamoto, T. Assignment of pre‐edge peaks in K‐edge X‐ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole? Xray Spectrom. 37, 572–584 (2008).
Kramm, U. I., Lefèvre, M., Larouche, N., Schmeisser, D. & Dodelet, J.-P. Correlations between mass activity and physicochemical properties of Fe/N/C catalysts for the ORR in PEM fuel cell via 57Fe Mössbauer spectroscopy and other techniques. J. Am. Chem. Soc. 136, 978–985 (2014).
Leonard, N. D. et al. Deconvolution of utilization, site density, and turnover frequency of Fe–nitrogen–carbon oxygen reduction reaction catalysts prepared with secondary N-precursors. ACS Catal. 8, 1640–1647 (2018).
Primbs, M. et al. Establishing reactivity descriptors for platinum group metal (PGM)-free Fe–N–C catalysts for PEM fuel cells. Energy Environ. Sci. 13, 2480–2500 (2020).
Mineva, T. et al. Understanding active sites in pyrolyzed Fe–N–C catalysts for fuel cell cathodes by bridging density functional theory calculations and 57Fe Mössbauer spectroscopy. ACS Catal. 9, 9359–9371 (2019).
Orellana, W. Catalytic properties of transition metal–N4 moieties in graphene for the oxygen reduction reaction: evidence of spin-dependent mechanisms. J. Phys. Chem. C 117, 9812–9818 (2013).
Sun, Y. et al. Itinerant ferromagnetic half metallic cobalt–iron couples: promising bifunctional electrocatalysts for ORR and OER. J. Mater. Chem. A 7, 27175–27185 (2019).
Sun, Y. et al. Spin-related electron transfer and orbital interactions in oxygen electrocatalysis. Adv. Mater. 32, 2003297 (2020).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 3, 546–550 (2011).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Xia, D. et al. Direct growth of carbon nanotubes doped with single atomic Fe–N4 active sites and neighboring graphitic nitrogen for efficient and stable oxygen reduction electrocatalysis. Adv. Funct. Mater. 29, 1906174 (2019).
Mun, Y. et al. Versatile strategy for tuning ORR activity of a single Fe-N4 site by controlling electron-withdrawing/donating properties of a carbon plane. J. Am. Chem. Soc. 141, 6254–6262 (2019).
Luo, F. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 19, 1215–1223 (2020).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Xiao, M. et al. Climbing the apex of the ORR volcano plot via binuclear site construction: electronic and geometric engineering. J. Am. Chem. Soc. 141, 17763–17770 (2019).
Gu, J., Hsu, C.-S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Wang, M. et al. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat. Commun. 10, 341 (2019).
Bo, J. et al. Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor. J. Power Sources 407, 105–111 (2018).
Li, P., Jin, Z., Fang, Z. & Yu, G. A single-site iron catalyst with preoccupied active center that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 14, 3522–3531 (2021).
Ahn, H. S. & Bard, A. J. Switching transient generation in surface interrogation scanning electrochemical microscopy and time-of-flight techniques. Anal. Chem. 87, 12276–12280 (2015).
Acknowledgements
G.Y. acknowledges the funding support from the US Department of Energy, Office of Science, Basic Energy Sciences under Award DE-SC0019019, the Welch Foundation Award F-1861 and the Camille Dreyfus Teacher-Scholar Award. We sincerely thank A.J. Bard and his group for the support and thoughtful discussion on SECM.
Author information
Authors and Affiliations
Contributions
G.Y. conceived and directed the project. Z.J. designed experiments, performed SI–SECM and simulations, and wrote the paper. P.L. conducted the synthesis of catalysts and electrochemical experiments. Y.M. and D.X. supported the characterizations and analysis. P.L. and Z.F. edited the manuscript. All authors discussed and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Hyun S. Park, Samira Siahrostami and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Tables 1–13, Notes 1–11, Methods and References.
Supplementary Data 1
Atomic coordinates.
Rights and permissions
About this article
Cite this article
Jin, Z., Li, P., Meng, Y. et al. Understanding the inter-site distance effect in single-atom catalysts for oxygen electroreduction. Nat Catal 4, 615–622 (2021). https://doi.org/10.1038/s41929-021-00650-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00650-w
This article is cited by
-
Two-electron redox chemistry via single-atom catalyst for reversible zinc–air batteries
Nature Sustainability (2024)
-
Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction
Nature Communications (2024)
-
Active site engineering accelerates water electrolysis
Nature Synthesis (2024)
-
Site-specific metal-support interaction to switch the activity of Ir single atoms for oxygen evolution reaction
Nature Communications (2024)
-
Current Status and Perspectives of Dual-Atom Catalysts Towards Sustainable Energy Utilization
Nano-Micro Letters (2024)